cobalt electron configuration|cobalt electron configuration full : Cebu The ground state electron configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. This electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. Therefore, the valence electrons of cobaltare nine. There are two types of cobalt ions. The . Tingnan ang higit pa FREE Hot Gay Porn Videos From MEN, Sean Cody, Corbin Fisher. to OnlyFans — Watch Free HD and Amateur Gay Porn Videos on FXGGXT.COM
PH0 · electron configuration for every element
PH1 · electron configuration calculator
PH2 · cobalt electron configuration unabbreviated
PH3 · cobalt electron configuration shorthand
PH4 · cobalt electron configuration long form
PH5 · cobalt electron configuration full
PH6 · cobalt electron configuration abbreviated
PH7 · cobalt 3+ electron configuration
PH8 · Iba pa
Unlocking the gateway to MWPLAY is an exciting milestone in your gambling journey. Once you’ve discovered our esteemed online casino, the next step is to navigate to the MWPLAY888 Login/Register page.. As a fully licensed establishment, we prioritize your security and adhere to the most stringent standards of gambling and data protection.
cobalt electron configuration*******The ground state electron configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. This electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. Therefore, the valence electrons of cobaltare nine. There are two types of cobalt ions. The . Tingnan ang higit pa
The total number of electrons in cobaltis twenty-seven. These electrons are arranged according to specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof the atom revolve around the nucleus in . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
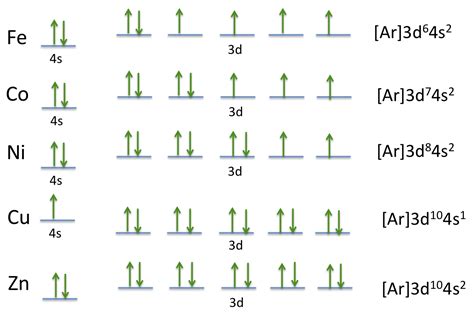
Mar 23, 2023 Full Electron Configuration For Cobalt. Full electron configuration can be defined as 27 electrons distribution in 4 shells of . To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of . In order to write the electron configuration we first need to know the number of electrons for the Cobalt (Co) atom. There are 27 electrons for the Cobalt atom. .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .

Learn how cobalt atoms arrange their 27 electrons in different orbitals and subshells, and how this affects their valence and valency. Find out the shorthand .Electron configuration 3d 7 4s 2: Electrons per shell: 2, 8, 15, 2: Physical properties; Phase at STP: solid: Melting point: 1768 K (1495 °C, 2723 °F) Boiling point: 3200 K (2927 °C, 5301 °F) Density (near r.t.) 8.90 g/cm 3: .
Cobalt electron configuration. ← Electronic configurations of elements. Co (Cobalt) is an element with position number 27 in the periodic table. Located in the IV period. . To write the orbital diagram for the Cobalt (Co) first we need to write the electron configuration for just Co. To do that we need to find the number of electrons .cobalt electron configuration full The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. Cobalt is a very hard, lustrous bluish grey metal that belongs to d-block and is under the category of transition metals. It is a hexagonal closed pack (hcp) crystal structure that is solid in normal conditions and is isolated by the process of smelting .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical .
The s,p,d,f configuration for cobalt (Co) is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7, determined by the position of the element on the periodic table. Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and therefore has 7 d electrons d^7. The element cobalt . Thus, the electron configuration for Cobalt at ground state would simply be Co: [Ar] 4s 2 3d 7. The reason why it is 3d 7 can be explained using the periodic table. As stated, you could simply count the boxes on the periodic table, and since Cobalt is the 7th element of the first row transition metals, we get Co: [Ar] 4s 2 3d 7.The electron configuration of Cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. Cobalt is a chemical element of the periodic table, it is located in group 9, its symbol is Co and its atomic number is 27. It is a ferromagnetic metal with a bluish and white hue, its Curie temperature is equal to 1388 degrees Kelvin.
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of . Cobalt (Co) lies with the transition metals on the periodic table. The atomic number of Cobalt is 27 with an atomic mass of 58.933195. Cobalt was first discovered in 1735 by George Brandt in Stockholm Sweden. It is used in many places today, such as, magnets materials, paint pigments, glasses, and even cancer therapy.
La configuration électronique complète du cobalt est 1s2 2s2 2p6 3s2 3p6 3d7 4s2, tandis que la configuration électronique simplifiée ou abrégée s'écrit [Ar]3d74s2. Grâce à la configuration Electron, il est possible de définir la manière dont les électrons possédés par les atomes d'un élément spécifique sont structurés. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Cobalt is [Ar] 3d7 4s2. Possible oxidation states are +2,3.
Electron configurations are written using the principal quantum number n, followed by the orbital (s, p, d, or f) with the total number of electrons written as a superscript. Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e .cobalt electron configuration To write the orbital diagram for the Cobalt (Co) first we need to write the electron configuration for just Co. To do that we need to find the number of ele.cobalt electron configuration cobalt electron configuration fullElectron configuration 3d 7 4s 2: Electrons per shell: 2, 8, 15, 2: . Cobalt is a chemical element; it has symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically .
The ground state electron configuration of cobalt is " [Ar]3d"^7"4s"^2. This noble gas notation means that cobalt has the electron configuration of argon plus the "3d"^7"4s"^2 electrons. Argon has an atomic number of 18, and a neutral atom has 18 electrons. Add the 18 electrons from argon plus the 9 additional electrons for cobalt, . Figure 5.16.1 5.16. 1 Order of filling subshells in the building-up of atomic electron configurations. (a) Relative energies of subshells at the time they are being filled. (b) Aid to remembering the order of filling subshells. All possible shells having the same n value are written on horizontal lines. Diagonal arrows from lower right to upper .
La configuration électronique du cobalt est [Ar] 3d7 4s2. Les états d’oxydation possibles sont +2,3. Les états d’oxydation courants du cobalt comprennent +2 et +3, bien que des composés avec des états d’oxydation allant de -3 à +5 soient également connus. Un état d’oxydation courant pour les composés simples est +2 (cobalt (II)).
Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .
Send unlimited free texts and make WiFi calls from a free phone number. Download the free app or sign up online to pick your free phone number.
cobalt electron configuration|cobalt electron configuration full